Light-induced reversible modification of the work function of a new perfluorinated biphenyl azobenzene chemisorbed on Au (111)†
Abstract
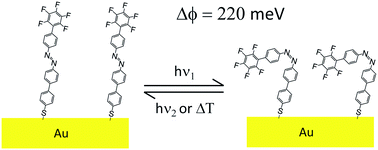
We describe the synthesis of a novel biphenyl azobenzene derivative exhibiting: (i) a protected thiol anchoring group in the α-position to readily form self-assembled monolayers (SAMs) on Au surfaces; and (ii) a terminal perfluorinated benzene ring in the ω-position to modify the surface properties. The design of this molecule ensured both an efficient in situ photoswitching between the trans and cis isomers when chemisorbed on Au(111), due to the presence of a biphenyl bridge between the thiol protected anchoring group and the azo dye, and a significant variation of the work function of the SAM in the two isomeric states, induced by the perfluorinated phenyl head group. By exploiting the light responsive nature of the chemisorbed molecules, it is possible to dynamically modify in situ the work function of the SAM-covered electrode, as demonstrated both experimentally and by quantum-chemical calculations, revealing changes in work function up to 220 meV. These findings are relevant for tuning the work function of metallic electrodes, and hence to dynamically modulate charge injection at metal–semiconductor interfaces for organic opto-electronic applications.

 Please wait while we load your content...
Please wait while we load your content...