Structural, magnetic and theoretical calculations of a ferromagnetically coupled tetranuclear copper(ii) square complex†
Abstract
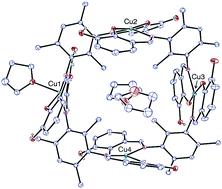
Condensation of 3-hydroxysalicylaldehyde with 2,4,6-trimethyl-1,3-phenylenediamine gives the ligand N,N′-bis(3-hydroxysalicylidene)-2,4,6-trimethyl-m-phenylenediamine (H4L). The dinuclear zinc(II) complex [Zn(H2L)]2 (1) and the tetranuclear copper(II) square complex [{Cu(H2L)}4(THF)] (2) were synthesized and structurally characterized by single crystal X-ray diffraction. In 1, the ZnII ions are bridged by two H2L molecules and they adopt a tetrahedral geometry. The coordination geometry of the copper atoms in 2 is either square planar or square pyramidal, each metal atom being bound to the NO donor sets of two Schiff base ligands and, in one case, to an extra THF molecule. The arrangement of the tetranuclear complexes in the crystal lattice results in the formation of square channels. Variable temperature magnetic measurements on 2 evidence significant long-range ferromagnetic interactions between the four CuII centres leading to an S = 2 ground state with J1 = +5.81, J2 = +2.36, J3 = +1.73 and J4 = +2.37 cm−1. DFT calculations have been carried out in order to corroborate the experimental fitting and ascertain the origin of this ferromagnetic behaviour.

 Please wait while we load your content...
Please wait while we load your content...