Binding studies of l-3,4-dihydroxyphenylalanine with human serum albumin
Abstract
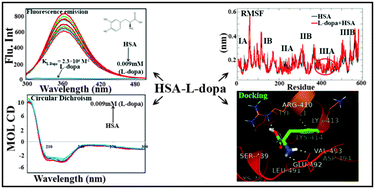
L-Dopa has been used to increase dopamine concentrations in the treatment of Parkinson's disease and dopamine-responsive dystonia. The binding interaction between L-dopa (phytochemical) and human serum albumin (HSA) under simulated physiological conditions was investigated by spectroscopic and molecular modeling methods. The results revealed that L-dopa caused fluorescence emission quenching of HSA through a static quenching procedure and the binding constant obtained was 2.3 ± 0.01 × 104 M−1, which is corresponding to −5.9 kcal M−1 of free energy at 25 °C. Interestingly, L-dopa is not binding to the α-1-acidglycoprotein, which is also a plasma protein and an acute phase protein. Furthermore, circular dichroism results confirm that in the presence of L-dopa the secondary structure of HSA is altered due to partial unfolding of the protein. Importantly, the displacement experiment with site specific probes, phenylbutazone (site I) and ibuprofen (site II), depicts that L-dopa binds particularly to site II of HSA. In addition, the molecular modeling results also confirmed that L-dopa is binding to the subdomain IIIA of HSA and is stabilized by hydrogen bonds and hydrophilic forces. Additionally, the molecular dynamic simulation studies showed that the HSA–L-dopa complex reaches an equilibration state at around 2 ns, which indicates that the HSA–L-dopa complex is very stable. These results provided valuable information of pharmacological mechanisms of L-dopa under in vivo conditions and play a pivotal role in the development of L-dopa-inspired drugs.

 Please wait while we load your content...
Please wait while we load your content...