Rice cadmium monitoring using heat-extraction electrothermal atomic absorption spectrometry
Abstract
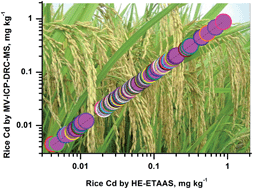
A simple and high throughput method involving heat-extraction electrothermal atomic absorption spectrometry was developed to monitor the Cd content in rice, and 184 rice samples purchased from supermarkets in Beijing were surveyed. The main advantages of using heat-extraction in comparison to slurry sampling are doubling of the graphite tube lifetime and 80% decrease in the background absorbance signal, most likely because the less sample matrix was introduced into the atomizer, avoiding buildup of carbonaceous residues or silicates on the graphite platform. Heat-extraction provides better detection limits and precision than microwave acid digestion. In the heat-extraction method, slurries in 3% v/v HNO3 were heated on a heating block. After centrifugation, the supernatant was introduced into a graphite tube pretreated with a W–Rh permanent modifier. The optimum conditions for Cd detection were 0.2–5.0% m/v slurry concentration, 3% HNO3 extract, 10 min heating at 120 °C, rice-particle size <150 μm (after 3 min of sample grinding), 600 °C pyrolysis temperature, and 1500 °C atomization temperature. The extraction recovery for Cd in rice standard reference materials was 98.4–101.1%. The characteristic masses and detection limit for Cd based on the integrated absorbance for a 2.5% m/v rice sample were 0.7 ± 0.1 pg and 1 ng g−1, respectively. The mean Cd content was 0.076 μg g−1 (ranging from 0.004 to 0.876 μg g−1), and only 4.3% of investigated rice samples exceeded the maximum allowable Cd concentration of 0.20 μg g−1. This simple and rapid method has great potential for monitoring trace Cd in food samples for market survey.

 Please wait while we load your content...
Please wait while we load your content...