Amplification of AngII-dependent cell contraction by glyoxal: implication of cell mechanical properties and actomyosin activity†
Abstract
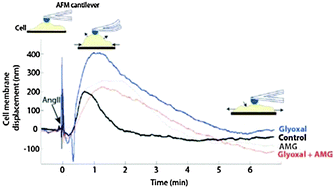
Glyoxal (GO), a highly reactive metabolite of glucose, is associated with diabetic vascular complications via the formation of advanced glycation end-products. Considering its ability to react with proteins' amino acids and its crosslinking potential, we suggest that GO affects cellular mechanical functions such as contractility. Therefore, we tested the effects of GO on cellular contractile response following AngII stimulation of human embryonic kidney cells over-expressing the AT1 receptor (HEK 293 AT1aR). Prior to cell stimulation with AngII, cells exposed to GO exhibited carboxymethyllysine-adduct formation and an increase in cellular stiffness, which could be prevented by pre-treatment with aminoguanidine. The time-dependent cellular contractile response to AngII was measured by monitoring cell membrane displacement by atomic force atomic force microscopy (AFM) and by quantifying myosin light chain phosphorylation (p-MLC) via immunoblotting. Interestingly, short-term GO exposure increased by 2.6 times the amplitude of cell contraction induced by AngII and this was also associated with a sustained rise in p-MLC. This increased response to AngII induced by GO appears to be linked to its glycation potential, as aminoguanidine pre-treatment prevented this increased cellular mechanical response. Our results also suggest that GO could have an impact on ROCK activity, as ROCK inhibition with Y-27632 blocked the enhanced contractile response (p = 0.011) measured under GO conditions. Together, these results indicate that GO enhances the cellular response to AngII and modifies cellular mechanical properties via a mechanism that relies on its glycation potential and on the activation of the ROCK-dependent pathway.

 Please wait while we load your content...
Please wait while we load your content...