STEP organic synthesis: an efficient solar, electrochemical process for the synthesis of benzoic acid†
Abstract
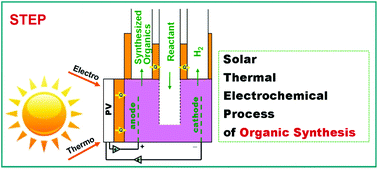
This study presents the first demonstration of STEP for organic synthesis. The synthesis of benzoic acid was fully driven by solar energy without the input of any other forms of energy. STEP (the Solar Thermal Electrochemical Process) was established to drive chemical reactions by using solar heat to minimize the energy and maximize the rate of a growing portfolio of electrolysis reactions. The use of solar energy can minimize or eliminate the carbon footprint associated with the production of societal staples. To date this has included STEP chemistries for the high solar efficiency generation of hydrogen fuels, carbon dioxide splitting to generate fuels and graphite, hematite (iron ore) splitting for iron metal, of lime from limestone, and the production of bleach, magnesium and other useful chemistries. Benzoic acid is produced by the electrolysis of toluene. Solar thermal and solar electrical energy synergistically drive the electrolysis; solar thermal decreases the requisite electrolysis voltage, and improves the kinetics, selectivity and yield of the reaction, while solar electrical current drives the electrolysis. The low electrolysis potential inhibits over-oxidation of the product. In this STEP organic synthesis of benzoic acid at an applied photopotential of 3 V the reaction conversion of benzoic acid is 3.9% at a temperature of 30 °C, 12.4% at 60 °C, and 32.0% at 90 °C. The results demonstrate that the STEP is suitable for an efficient synthesis of benzoic acid from toluene.

 Please wait while we load your content...
Please wait while we load your content...