A robust and active hybrid catalyst for facile oxygen reduction in solid oxide fuel cells†
Abstract
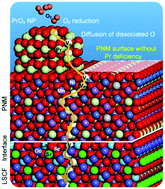
The sluggish oxygen reduction reaction (ORR) greatly reduces the energy efficiency of solid oxide fuel cells (SOFCs). Here we report our findings in dramatically enhancing the ORR kinetics and durability of the state-of-the-art La0.6Sr0.4Co0.2Fe0.8O3 (LSCF) cathode using a hybrid catalyst coating composed of a conformal PrNi0.5Mn0.5O3 (PNM) thin film with exsoluted PrOx nanoparticles. At 750 °C, the hybrid catalyst-coated LSCF cathode shows a polarization resistance of ∼0.022 Ω cm2, about 1/6 of that for a bare LSCF cathode (∼0.134 Ω cm2). Further, anode-supported cells with the hybrid catalyst-coated LSCF cathode demonstrate remarkable peak power densities (∼1.21 W cm−2) while maintaining excellent durability (0.7 V for ∼500 h). Near Ambient X-ray Photoelectron Spectroscopy (XPS) and Near Edge X-Ray Absorption Fine Structure (NEXAFS) analyses, together with density functional theory (DFT) calculations, indicate that the oxygen-vacancy-rich surfaces of the PrOx nanoparticles greatly accelerate the rate of electron transfer in the ORR whereas the thin PNM film facilitates rapid oxide-ion transport while drastically enhancing the surface stability of the LSCF electrode.


 Please wait while we load your content...
Please wait while we load your content...