On the coordination non-innocence of antimony in nickel(ii) complexes of the tetradentate (o-(Ph2P)C6H4)3Sb ligand†
Abstract
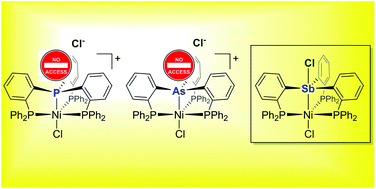
In a continuation of our investigations into the unusual properties of antimony-based ligands, we have decided to investigate the structure of the complexes formed by the tetradentate ligands (o-(Ph2P)C6H4)3E (E = P (1), As (2), Sb (3)) with NiCl2. We observed that the structure of the resulting complexes depends on the nature of the central pnictogen atom E, with the phosphorus- and arsenic-based systems adopting ionic structures of the type [ClNi(o-(Ph2P)C6H4)3E][Cl] (E = P ([4][Cl]), As ([5][Cl])) while the known antimony complex ClNi(o-(Ph2P)C6H4)3SbCl (6-Cl) is molecular, with a chloride anion bound to the antimony atom trans from the nickel atom. Using both structural and computational arguments, we propose that the Lewis acidic behavior of the antimony atom in the cation [ClNi(o-(Ph2P)C6H4)3Sb]+ ([6]+) which as been structurally characterized as its [BPh4]− salt, originates in part from the steric compression imposed by the rigid ligand buttresses and the larger size of antimony. This steric compression pushes the antimony atom of [6]+ toward a geometry that is closer to that of a trigonal-base pyramid, setting the stage for the coordination of a ligand trans from the nickel atom as in 6-Cl. Coordination of the chloride ligand also benefits from an accumulation of positive character at the antimony atom of [6]+ which is a assigned to the polarizable and electron-releasing nature of this heavy pnictogen.
- This article is part of the themed collections: Recipients of the Dalton Transactions UC Berkeley Lecture and Multimetallic complexes: synthesis and applications


 Please wait while we load your content...
Please wait while we load your content...