Structure and photoluminescence of silver(i) trinuclear halopyrazolato complexes†
Abstract
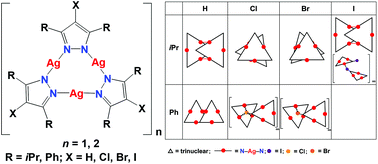
Five halogen substituted pyrazolates, 4-chloro-3,5-diisopropylpyrazole (4-Cl-3,5-iPr2pzH), 4-bromo-3,5-diisopropylpyrazole (4-Br-3,5-iPr2pzH), 4-iodo-3,5-diisopropylpyrazole (4-I-3,5-iPr2pzH), 4-chloro-3,5-diphenylpyrazole (4-Cl-3,5-Ph2pzH), and 4-bromo-3,5-diphenylpyrazole (4-Br-3,5-Ph2pzH), were conveniently prepared by halogenation of the appropriate pyrazoles with N-halosuccinimides (NXS) (X = Cl, Br, and I) followed by complexation of the pyrazolate anions with silver(I) nitrate. Single crystal X-ray analysis revealed either dimeric trinuclear {[Ag(μ-4-X-3,5-R2pz)]3}2 (R = iPr, X = Cl, Br, and I) or trinuclear [Ag(μ-4-X 3,5-R2pz)]3 (R = iPr, X = I; R = Ph, X = Cl, R = Ph, X = Br) structures, the latter held together with argentophilic interactions (Ag⋯Ag interactions) that could also be observed in the Raman spectra. The electronegativity of the halogen substituent could be correlated with the strength of the Ag⋯Ag interaction and the wavelength of solid-state photoluminescence. All complexes were emissive on UV irradiation at low temperatures, with the colour of emission from the diisopropyl substituted analogues red shifted by the halogens in the order Cl (red) > Br (orange) > I (yellow). Emission from the diphenyl substituted analogues was dominated by the extended aromatic system and was largely invariant to the halogens.

 Please wait while we load your content...
Please wait while we load your content...