Ferrocenylpyrazolyl palladium complexes as catalysts for the polymerisation of 1-heptene and 1-octene to highly branched polyolefins†
Abstract
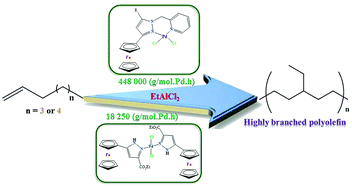
Reactions of [PdCl2(NCMe)2] with the ferrocenylpyrazolyl compounds: 3-ferrocenyl-1H-pyrazole-5-carboxylate (L1), ethyl-1-(2-bromoethyl)-3-ferrocenyl-1H-pyrazole-5-carboxylate (L2a), ethyl-1-(2-bromoethyl)-5-ferrocenyl-1H-pyrazole-3-carboxylate (L2b), 3-ferrocenylpyrazolyl-methylenepyridine (L3) and 3-ferrocenyl-5-methylpyrazolyl-methylenepyridine (L4) at room temperature afforded [PdCl2(L1)] (1), [PdCl2(L2a)] (2a), [PdCl2(L2b)] (2b), [PdCl2(L3)] (3) and [PdCl2(L4)] (4) respectively. Compounds L1–L4 and their palladium complexes were obtained in moderate to high (50–90%) yield and characterized by NMR spectroscopy, elemental analysis, and in selected cases by single crystal X-ray crystallography. Complexes 1–4 were used as pre-catalysts for the reactions of 1-heptene and 1-octene with EtAlCl2 as a co-catalyst. The activities of 1, 2a and 3b were relatively low (10 083–18 250 g molPd−1 h−1) with 1-heptene being a better monomer for these complexes. However, pre-catalysts 3 and 4 showed moderate activities (123 000–448 000 g molPd−1 h−1) 1-octene being the better monomer. The polyolefins obtained were characterized by both 1H and 13C{1H} NMR to be highly branched polyolefins with degree of branching up to 270 branches per 1000 carbon atoms. However, low molecular weights between 888 and 1198 were recorded with narrow PDI between 1.02 and 1.44.

 Please wait while we load your content...
Please wait while we load your content...