Bis-tropolonate complexes of tungsten: scaffolds for selective side-on binding of nitriles, imines and ketones†
Abstract
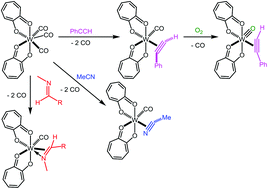
The chiral bis-tropolonate tungsten(II) tricarbonyl compound, (trop)2W(CO)3 (1), has been synthesized and structurally characterized. This seven-coordinate compound readily loses two carbonyl ligands to preferentially bind a series of π-bonding substrates to form six-coordinate complexes of the type (trop)2W(CO)(L). Alkynes coordinate strongly to form (trop)2W(CO)(η2-RCCR) (2) in which spectroscopic data is consistent with the alkyne serving as a 4-electron donor. Compound 1 will also preferentially coordinate organic nitriles in a side-on fashion through the CN triple bond. A dramatic shift in the nitrile carbon signals to greater than 210 ppm in the 13C NMR confirms the nitriles are coordinated in an η2 4-electron donating capacity. Aldehydes, ketones, and imines also react with 1 to form 4-electron donor η2 adducts. The imine adduct (trop)2W(CO)(η2-MeN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)(tol)) (5) was characterized crystallographically and the short 1.91 A W–N bond distance supports the postulation of 4-electron donation from the imine through C
C(H)(tol)) (5) was characterized crystallographically and the short 1.91 A W–N bond distance supports the postulation of 4-electron donation from the imine through C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N π-bonding and N lone pair donation. Side-on coordination of ligands of this type is rare and may provide a means towards asymmetric functionalization of these substrates. All of the tropolonate compounds are prone to oxidation in air and the alkyne compounds will oxidize to stable WIV oxo alkyne species, (trop)2W(O)(η2-RCCR) (6). This causes a 90° rotation of the alkyne ligand and a reduction in alkyne donation to approximately 3 electrons, to maintain an optimal 18 electron configuration.
N π-bonding and N lone pair donation. Side-on coordination of ligands of this type is rare and may provide a means towards asymmetric functionalization of these substrates. All of the tropolonate compounds are prone to oxidation in air and the alkyne compounds will oxidize to stable WIV oxo alkyne species, (trop)2W(O)(η2-RCCR) (6). This causes a 90° rotation of the alkyne ligand and a reduction in alkyne donation to approximately 3 electrons, to maintain an optimal 18 electron configuration.

 Please wait while we load your content...
Please wait while we load your content...