Redox and complexation chemistry of the CrVI/CrV-d-glucaric acid system†
Abstract
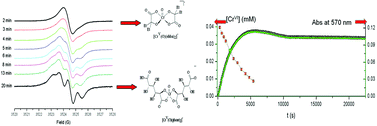
When an excess of uronic acid over CrVI is used, the oxidation of D-glucaric acid (Glucar) by CrVI yields D-arabinaric acid, CO2 and CrIII-Glucar complex as final redox products. The redox reaction involves the formation of intermediate CrIV and CrV species. The reaction rate increases with [H+] and [substrate]. The experimental results indicated that CrIV and CrV are very reactive intermediates since their disappearance rates are much faster than CrVI. CrIV and CrV intermediates are involved in fast steps and do not accumulate in the redox reaction of the mixture CrVI–Glucar. Kinetic studies show that the redox reaction between Glucar and CrVI proceeds through a mechanism combining one- and two-electron pathways: CrVI → CrIV → CrII and CrVI → CrIV → CrIII. After the redox reaction, results show a slow hydrolysis of the CrIII-Glucar complex into [Cr(OH2)6]3+. The proposed mechanism is supported by the observation of free radicals, CrO22+ (superoxo-CrIII ion) and oxo-CrV-Glucar species as reaction intermediates. The continuous-wave electron paramagnetic resonance, CW-EPR, spectra show that five-coordinate oxo-CrV bischelates are formed at pH ≤ 4 with the aldaric acid bound to oxo-CrV through the carboxylate and the α-OH group. A different oxo-CrV species with Glucar was detected at pH 6.0. The high giso value for the last species suggests a mixed coordination species, a five-coordinated oxo-CrV bischelate with one molecule of Glucar acting as a bi-dentate ligand, using the 2-hydroxycarboxylate group, and a second molecule of Glucar with any vic-diolate sites. At pH 7.5 only a very weak EPR signal was observed, which may point to instability of these complexes. This behaviour contrasts with oxo-CrV-uronic species, and must thus be related to the Glucar acyclic structure. In vitro, our studies on the chemistry of oxo-CrV-Glucar complexes can provide information on the nature of the species that are likely to be stabilized in vivo.

 Please wait while we load your content...
Please wait while we load your content...