The usefulness of EPR spectroscopy in the study of compounds with metal–metal multiple bonds†
Abstract
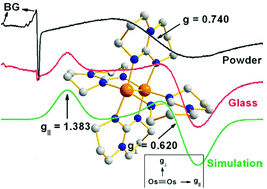
This Perspective reviews some of the contributions that EPR spectroscopy has made for the understanding of the bonding and electronic structure of molecular species with metal-to-metal multiple bonds. The goal is to show how useful this technique can be in (a) elucidating whether unpaired electrons reside in metal-based or ligand-based molecular orbitals, (b) providing information on the metal center's total electronic spin, an aspect that offers support to its oxidation state, (c) informing about the distribution of the unpaired electrons between the metals and the organic ligand, (d) detecting molecular dynamics and phase transitions to complement X-ray studies and (e) using high-frequency, high-field EPR for studying compounds that might be EPR-silent when only commonly available spectrometers are used. A brief comparison with NMR is provided in the ESI where it is noted that for typical laboratory magnetic fields, the energy gap between resonant states in EPR is in the microwave region of the electromagnetic spectrum (GHz) while for NMR it is in the radiofrequency range (MHz). Our aim is to encourage the inorganic chemistry community and more broadly other chemists and physicists to collaborate while characterizing new paramagnetic inorganic complexes. Enough basic background is provided as ESI to allow senior-level undergraduate and graduate students to understand the simplicity as well as the power of EPR spectroscopy with a view to encourage them to use this technique in their own research activities. Finally, it should be noted that this report commemorates the fiftieth anniversary of the description in Science of the first species with a quadruple bond.

 Please wait while we load your content...
Please wait while we load your content...