Molecular and electronic structure of nonradical homoleptic pyridyl-azo-oxime complexes of cobalt(iii) and the azo-oxime anion radical congener: an experimental and theoretical investigation†
Abstract
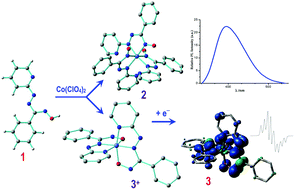
The reaction between a potential flexidentate pyridyl-azo-oxime HL 1 and Co(ClO4)2 yields novel homoleptic complexes of types [CoIII(L−I)3], 2 and [CoIII(L−I)2]ClO4, 3+ClO4 in N6 and N4O2 coordination environments respectively. The FMOs of these complexes vary appreciably and are strongly modified by the coordination environment. This has striking influences on the spectral and redox properties of the metallo conjugates of ligand HL. The synthesized bis 2 and tris chelates 3+ possess well-defined optoelectronic and redox properties and these are scrutinized by the density functional theory (DFT) and time dependent density functional theory (TD-DFT) analyses. The visible excitations are primarily mixed singlet-manifold 1ILCT and 1LLCT transitions, with different amounts of ligand π–π* character while in the UV region, the excitations are essentially π–π* ILCT/LLCT transitions for the 3+ and ILCT/LLCT transitions along with the LMCT component for 2. The luminescent cobalt(III) species are rarely cited albeit these are found to be moderately blue emissive with slight quenching of the emission quantum yield (Φ) as compared to that of a free ligand. Computation reveals that the cobalt d orbital is involved in the triplet emissive excited states and this phenomenon is plausibly responsible for the quenching of the emission quantum yield in the complexes. Both types of complexes are electro-active in solution and the first reductive response, associated with the redox orbital comprising delocalized π orbital of a ligand, is shifted in the more positive potential (0.6 V) in 3+ relative to 2 and this observation is corroborated with the appreciable stabilization (∼0.5 eV) of LUMO of 3+ (coordination mode A) as compared to that in 2 (coordination mode B). This provides us an opportunity to explore the cobalt-bound azo-oxime anion radical compound by reduction of the diamagnetic precursor 3+. The best description of the one-electron paramagnetic 3 can be ascertained as [CoIII{(L−I)2}˙−] from the EPR and DFT studies where the unpaired spin is delocalized essentially over π* orbital comprising both the coordinated ligands (97%) with little participation of cobalt dyz (3%).

 Please wait while we load your content...
Please wait while we load your content...