Homo- and heteropolymetallic 3-(2-pyridyl)pyrazolate manganese and rhenium complexes†
Abstract
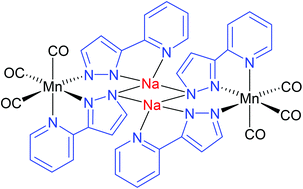
fac-[MBr(CO)3(pypzH)] (M = Mn, Re; pypzH = (3-(2-pyridyl)pyrazole) complexes are prepared from fac-[MBr(CO)3(NCMe)2] and pypzH. The result of their deprotonation depends on the metallic substrate: the rhenium complex affords cleanly the bimetallic compound [fac-{Re(CO)3(μ2-pypz)}]2 (μ2-pypz = μ2-3-(2-pyridyl-κ1N)pyrazolate-2κ1N), which was crystallographically characterized, whereas a similar manganese complex was not detected. When two equivalents of pyridylpyrazolate are used, polymetallic species [fac-M(CO)3(μ2-pypz)(μ3-pypz)M′] (μ3-pypz = μ3-3-(2-pyridyl-κ1N)pyrazolate-1κ2N,N:2κ1N:; M = Mn, M′ = Li, Na, K; M = Re, M′ = Na) are obtained. The crystal structures of the manganese carbonylate complexes were determined. The lithium complex is a monomer containing one manganese and one lithium atom, whereas the sodium and potassium complexes are dimers and reveal an unprecedented coordination mode for the bridging 3-(2-pyridyl)pyrazolate ligand, where the nitrogen of the pyridyl fragment and the nitrogen-1 of pyrazolate are chelated to manganese atoms, and each nitrogen-2 of pyrazolate is coordinated to two alkaline atoms. The polymetallic carbonylate complexes are unstable in solution and evolve spontaneously to [fac-{Re(CO)3(μ2-pypz)}]2 or to the trimetallic paramagnetic species [MnII(μ2-pypz)2{fac-{MnI(CO)3(μ2-pypz)}2}]. The related complex cis-[MnCl2(pypzH)2] was also synthesized and structurally characterized. The electrochemical behavior of the new homo- and heteropolymetallic 3-(2-pyridyl)pyrazolate complexes has been studied and details of their redox properties are reported.

 Please wait while we load your content...
Please wait while we load your content...