Synthesis, stereocontrol and structural studies of highly luminescent chiral tris-amidepyridyl-triazacyclononane lanthanide complexes†
Abstract
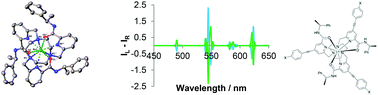
The configuration of the remote amide chiral moiety determines the helicity of the metal complex in Ln(III) complexes of nonadentate N6O3 ligands based on triazacyclononane. Solution NMR studies revealed the presence of a dominant isomer whose proportion varies from 9 : 1 to 4 : 1 from Ce to Yb and X-ray crystallographic studies at 120 K of the Yb and two enantiomeric Eu complexes confirmed the configuration as S-Δ-λ in the major isomer. Global minimisation methods allowed magnetic susceptibility and electronic relaxation times of the lanthanide ions to be estimated by analysis of variable field longitudinal relaxation rate (R1) data sets. A set of four europium complexes, containing different para-substituted pyridinyl-aryl groups, exist as one major isomer (15 : 1), and absorb light strongly via an ICT transition in the range 320 to 355 nm (ε = 55 to 65 000 M−1 cm−1). The two examples absorbing light at 332 nm, possess overall emission quantum yields of 35 and 37% in aerated water, making these systems as bright as any Eu complex in solution.

 Please wait while we load your content...
Please wait while we load your content...