Radical coupling for directed C–C/C–S bond formation in the reaction of Cp*IrS2C2B10H10 with 1-azido-3-nitrobenzene†
Abstract
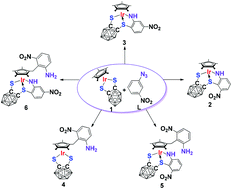
Reactions of half-sandwich complex Cp*IrS2C2B10H10 (1) with 1-azido-3-nitrobenzene (3-NO2C6H4N3, L) upon heating or under light led to new complexes 2–6. Complexes 2 and 3 contain a five-membered cyclometalated ligand arising from C(sp2)–H activation of the azide ligand L. Complex 4 is a 16 electron species containing a new-generated C–C bond between the azide ligand L and the Cp* unit where C(sp3)–H activation of the methyl unit occurred. Complexes 5 and 6 contain two types of the ligand which appear in complexes 2, 3 and 4. Further reactions of complexes 5 and 6 with L under more harsh conditions gave rise to the nucleophilic addition products 7 and 8, where ring expansion of the azide ligand at the imido site of complexes 5 and 6 happened. Complexes 2–8 were characterized by NMR, MS, IR, and elemental analysis, and X-ray structural analyses were performed for complexes 2–4 and 6–8. The radical mechanisms for the formation of complexes 2–6 were proposed on the basis of capture experiments by EPR and ESI-MS. And the formation mechanism of complexes 7 and 8 was also suggested.
- This article is part of the themed collection: Carboranes

 Please wait while we load your content...
Please wait while we load your content...