Acceptorless dehydrogenative coupling of primary alcohols to esters by heterogeneous Pt catalysts†
Abstract
Supported platinum catalysts have been studied for the acceptor-free dehydrogenative coupling of primary alcohols to esters in the liquid phase under solvent-free conditions in N2 at 180 °C. The activity depends on the support material, and Pt-loaded SnO2 (Pt/SnO2) gives the highest activity. Pt/SnO2 shows higher activity than various transition metals (Ir, Re, Ru, Rh, Pd, Ag, Co, Ni, Cu) loaded on SnO2. The Pt/SnO2 catalyst (1 mol%) selectively converted various primary alcohols to their corresponding esters in moderate to high isolated yield (53–91%). This is the first example of reusable heterogeneous catalysts for the acceptor-free dehydrogenative coupling of primary alcohols to esters under additive-free and solvent-free conditions. Mechanistic and infrared (IR) studies are also shown to discuss the reaction pathway and a possible role of the SnO2 support as Lewis acid sites that activate carbonyl groups of adsorbed aldehyde intermediates.
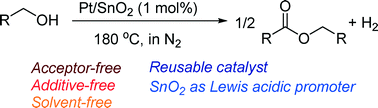

 Please wait while we load your content...
Please wait while we load your content...