Vapor phase hydrodeoxygenation of furfural to 2-methylfuran on molybdenum carbide catalysts†
Abstract
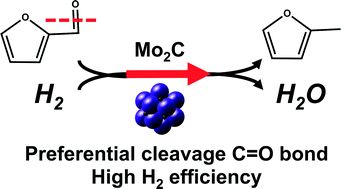
Vapor phase hydrodeoxygenation (HDO) of furfural over Mo2C catalysts at low temperatures (423 K) and ambient pressure showed high/low selectivity to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond/C–C bond cleavage, resulting in selectivity to 2-methylfuran (2MF) and furan of ~50–60% and <1%, respectively. Efficient usage of H2 for deoxygenation, instead of unwanted sequential hydrogenation, was evidenced by the low selectivity to 2-methyltetrahydrofuran. The apparent activation energy and H2 order for 2MF production rates were both found to be invariant with furfural conversion caused by catalyst deactivation, suggesting that (1) the measured reaction kinetics are not influenced by the products of furfural HDO and (2) the loss of active sites, presumably by formation of carbonaceous species observed by TEM analysis, is the reason for the observed catalyst deactivation. The observed half order dependence of 2MF production rates on H2 pressure at different furfural pressures (~0.12–0.96 kPa) and the 0–0.3 order dependence in furfural pressure support the idea of two distinct sites required for vapor phase furfural HDO reactions on Mo2C catalysts. The invariance of 2MF production rates normalized by the number of catalytic centers assessed via ex situ CO chemisorption suggests that metal-like sites on Mo2C catalysts are involved in selective HDO reactions.
O bond/C–C bond cleavage, resulting in selectivity to 2-methylfuran (2MF) and furan of ~50–60% and <1%, respectively. Efficient usage of H2 for deoxygenation, instead of unwanted sequential hydrogenation, was evidenced by the low selectivity to 2-methyltetrahydrofuran. The apparent activation energy and H2 order for 2MF production rates were both found to be invariant with furfural conversion caused by catalyst deactivation, suggesting that (1) the measured reaction kinetics are not influenced by the products of furfural HDO and (2) the loss of active sites, presumably by formation of carbonaceous species observed by TEM analysis, is the reason for the observed catalyst deactivation. The observed half order dependence of 2MF production rates on H2 pressure at different furfural pressures (~0.12–0.96 kPa) and the 0–0.3 order dependence in furfural pressure support the idea of two distinct sites required for vapor phase furfural HDO reactions on Mo2C catalysts. The invariance of 2MF production rates normalized by the number of catalytic centers assessed via ex situ CO chemisorption suggests that metal-like sites on Mo2C catalysts are involved in selective HDO reactions.
- This article is part of the themed collection: Sustainable catalytic conversions of renewable substrates

 Please wait while we load your content...
Please wait while we load your content...