An investigation on surface reactivity of Nb-doped vanadyl pyrophosphate catalysts by reactivity experiments and in situ Raman spectroscopy
Abstract
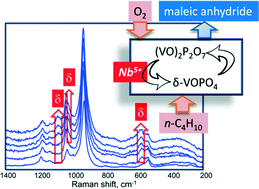
The effect of Nb when used as a promoter for vanadyl pyrophosphate, the catalyst for the oxidation of n-butane to maleic anhydride, was investigated. By means of both reactivity experiments and in situ Raman spectroscopy, it was found that the promoting effect shown by Nb occurred already for a very low amount of it, i.e., for a V/Nb atomic ratio as high as 150. The main role of Nb was to accelerate the formation of a limited amount of δ-VOPO4 on the surface of vanadyl pyrophosphate, by prompting the oxidation of V4+ into V5+ under reaction conditions. On the other hand, the presence of Nb concentrations higher than the optimal amount led to the generation of an undesired excess quantity of VOPO4 and the formation of αII-VOPO4, as well as having a detrimental effect on the catalytic behavior.

 Please wait while we load your content...
Please wait while we load your content...