Imidazolium-based ionic liquids grafted on solid surfaces
Abstract
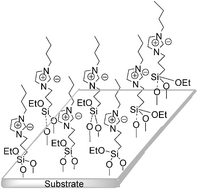
Supported ionic liquids (SILs), which refer to ionic liquids (ILs) immobilized on supports, are among the most important derivatives of ILs. The immobilization process of ILs can transfer their desired properties to substrates. Combination of the advantages of ILs with those of support materials will derive novel performances while retaining properties of both moieties. SILs have been widely applied in almost all of fields involving ILs, and have brought about drastic expansion of the ionic liquid area. As green media in organic catalytic reactions, based on utilizing the ability of ILs to stabilize the catalysts, they have many advantages over free ILs, including avoiding the leaching of ILs, reducing their amount, and improving the recoverability and reusability of both themselves and catalysts. This has critical significance from both environmental and economical points of view. As novel functional materials in surface science and material chemistry, SILs are ideal surface modifying agents. They can modify and improve the properties of solids, such as wettability, lubricating property, separation efficiency and electrochemical response. With the achievements in the field of ILs, using magnetic nanoparticles (MNPs) to SILs has drawn increasing attention in catalytic reactions and separation technologies, and achieved substantial progress. The combination of MNPs and ILs renders magnetic SILs, which exhibit the unique properties of ILs as well as facile separation by an external magnetic field. In this article, we focus on imidazolium-based ILs covalently grafted to non-porous and porous inorganic materials. The excellent stability and durability of this kind of SILs offer a great advantage compared with free ILs and IL films physically adsorbed on substrates without covalent bonds. Including examples from our own research, we overview mainly the applications and achievements of covalent-linked SILs in catalytic reactions, surface modification, separation technologies and electrochemistry.

 Please wait while we load your content...
Please wait while we load your content...