Double-stranded dimetallic helicates: assembling–disassembling driven by the CuI/CuII redox change and the principle of homochiral recognition
Abstract
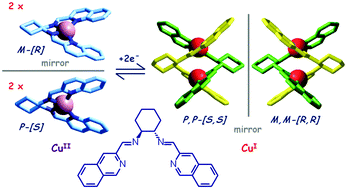
In the presence of d10 metal ions, prone to tetrahedral coordination, ligands containing two bidentate subunits will give rise to double-stranded helical complexes (helicates). Upon electrochemical oxidation of CuI to CuII, the helicate complex tends to disassemble, thus giving rise to two mononuclear CuII complexes with tetragonal geometry. Upon subsequent CuII-to-CuI electrochemical reduction, two CuI complexes instantaneously re-assemble to give the helicate complex. A helicand containing a chiral subunit (e.g. 1,2-substituted cyclohexanediamine) contains a racemic mixture of the R,R and S,S enantiomers. The racemic helicand, reacting with CuI, forms dimetallic helicates, in which the two strands show the same chirality, whether R,R or S,S, thus obeying the principle of homochiral recognition.
- This article is part of the themed collection: Supramolecular and dynamic covalent reactivity

 Please wait while we load your content...
Please wait while we load your content...