Transition states of spin-forbidden reactions†
Abstract
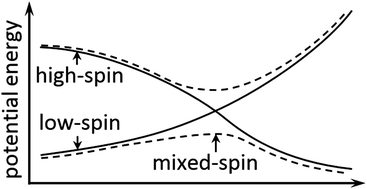
Spin–orbit coupling plays an important role in determining the mechanisms and kinetics of spin-forbidden reactions and many reactions exhibiting two-state reactivity. Spin–orbit coupling can allow the system to change its spin state, especially when potential energy surfaces (PESs) of two spin states approach each other. Here, we introduce a convenient new approximation method for locating stationary points on the lowest mixed-spin potential energy surface along a reaction pathway by using density functional calculations. The mixing of different spin states is achieved by introducing the spin–orbit coupling into the electronic Hamiltonian using a pre-defined coupling constant. Two examples are given using the new methodology: (a) a CO association reaction with the coordinatively unsaturated Fe(CO)4 complex and (b) an α-H elimination reaction of a model complex containing W. We computed a Gibbs free energy of activation of 2.8 kcal mol−1 for the CO association reaction, which is reasonably consistent with the experimentally measured reaction rate. For the H elimination reaction, the spin change occurs at a relatively low energy, and the present treatment allows one conclude that kinetics of the reaction can be reasonably well described without spin–orbit coupling.


 Please wait while we load your content...
Please wait while we load your content...