The staging mechanism of AlCl4 intercalation in a graphite electrode for an aluminium-ion battery†
Abstract
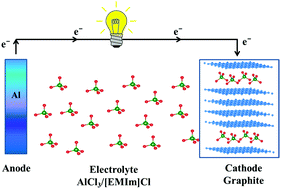
Identifying a suitable electrode material with desirable electrochemical properties remains a primary challenge for rechargeable Al-ion batteries. Recently an ultrafast rechargeable Al-ion battery was reported with high charge/discharge rate, (relatively) high discharge voltage and high capacity that uses a graphite-based cathode. Using calculations from first-principles, we have investigated the staging mechanism of AlCl4 intercalation into bulk graphite and evaluated the stability, specific capacity and voltage profile of AlCl4 intercalated compounds. Ab initio molecular dynamics is performed to investigate the thermal stability of AlCl4 intercalated graphite structures. Our voltage profiles show that the first AlCl4 intercalation step could be a more sluggish step than the successive intercalation steps. However, the diffusion of AlCl4 is very fast in the expanded graphite host layers with a diffusion barrier of ∼0.01 eV, which justifies the ultrafast charging rate of a graphite based Al-ion battery. And such an AlCl4 intercalated battery provides an average voltage of 2.01–2.3 V with a maximum specific capacity of 69.62 mA h g−1, which is excellent for anion intercalated batteries. Our density of states and Bader charge analysis shows that the AlCl4 intercalation into the bulk graphite is a charging process. Hence, we believe that our present study will be helpful in understanding the staging mechanism of AlCl4 intercalation into graphite-like layered electrodes for Al-ion batteries, thus encouraging further experimental work.


 Please wait while we load your content...
Please wait while we load your content...