Diffusional transport to and through thin-layer nanoparticle film modified electrodes: capped CdSe nanoparticle modified electrodes†
Abstract
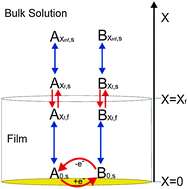
We present a simple and general theoretical model which accounts fully for the influence of an electrode modifying non-electroactive layer on the voltammetric response of a diffusional redox probe. The layer is solely considered to alter the solubilities and diffusion coefficients of the electroactive species within the thin layer on the electrode surface. On this basis it is demonstrated how, first, the apparent electrochemical rate constant can deviate significantly from that measured at an unmodified electrode. Second, depending on the conditions within the layer the modification of the electrode may lead to either apparent ‘negative’ or ‘positive’ electrocatalytic effects without the true standard electrochemical rate constant for the electron transfer at the electrode surface being altered. Having presented the theoretical model three experimental cases are investigated, specifically, the reductions of ruthenium(III) hexaamine, oxygen and boric acid on a gold macro electrode with and without a multi-layer organic capped nanoparticle film. In the latter case of the reduction of boric acid the voltammetric reduction is found to be enhanced by the presence of the organic layer. This result is interpreted as being due to an increase in the solubility of the analyte within the non-electroactive layer and not due to an alteration of the standard electrochemical rate constant.

 Please wait while we load your content...
Please wait while we load your content...