Introducing manganese complexes as redox mediators for dye-sensitized solar cells†
Abstract
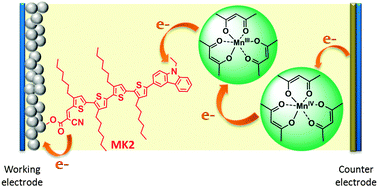
The abundance and low toxicity of manganese have led us to explore the application of manganese complexes as redox mediators for dye sensitized solar cells (DSCs), a promising solar energy conversion technology which mimics some of the key processes in photosynthesis during its operation. In this paper, we report the development of a DSC electrolyte based on the tris(acetylacetonato)manganese(III)/(IV), [Mn(acac)3]0/1+, redox couple. PEDOT-coated FTO glass was used as a counter electrode instead of the conventionally used platinum. The influence of a number of device parameters on the DSC performance was studied, including the concentration of the reduced and oxidized mediator species, the concentration of specific additives (4-tert-butylpyridine, lithium tetrafluoroborate, and chenodeoxycholic acid) and the thickness of the TiO2 working electrode. These studies were carried out with a new donor–π–acceptor sensitizer K4. Maximum energy conversion efficiencies of 3.8% at simulated one Sun irradiation (AM 1.5 G; 1000 W m−2) with an open circuit voltage (VOC) of 765 mV, a short-circuit current (JSC) of 7.8 mA cm−2 and a fill factor (FF) of 0.72 were obtained. Application of the commercially available MK2 and N719 sensitizers resulted in an energy conversion efficiency of 4.4% with a VOC of 733 mV and a JSC of 8.6 mA cm−2 for MK2 and a VOC of 771 mV and a JSC of 7.9 mA cm−2 for N719. Both dyes exhibit higher incident photon to current conversion efficiencies (IPCEs) than K4.
- This article is part of the themed collection: Photosynthesis: From Natural to Artificial

 Please wait while we load your content...
Please wait while we load your content...