The electrochemical reduction of 1-bromo-4-nitrobenzene at zinc electrodes in a room-temperature ionic liquid: a facile route for the formation of arylzinc compounds
Abstract
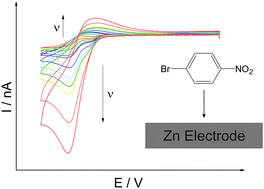
The electrochemical reduction of 1-bromo-4-nitrobenzene (p-BrC6H4NO2) at zinc microelectrodes in the [C4mPyrr][NTf2] ionic liquid was investigated via cyclic voltammetry. The reduction was found to occur via an EC type mechanism, where p-BrC6H4NO2 is first reduced by one electron, quasi-reversibly, to yield the corresponding radical anion. The radical anions then react with the Zn electrode to form arylzinc products. Introduction of carbon dioxide into the system led to reaction with the arylzinc species, fingerprinting the formation of the latter. This method thus demonstrates a proof-of-concept of the formation of functionalised arylzinc species.

 Please wait while we load your content...
Please wait while we load your content...