A structural investigation of novel thiophene-functionalized BEDT-TTF donors for application as organic field-effect transistors†
Abstract
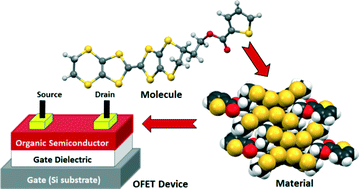
Three new unsymmetrical thiophene-functionalized bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF) donors (1–3) have been synthesized, characterised and examined as semiconducting materials for organic field-effect transistor (OFET) devices. The X-ray crystal structures of (1) and (2) reveal both neutral donors pack as dimers with lateral S⋯S contacts. For (1) the molecules are co-facially stacked in a head-to-tail manner with some degree of latitudinal slippage. A device prepared from a crystalline thin film of (1) deposited on unmodified silicon wafer substrate displays a mobility of 5.9 × 10−3 cm2 V−1 s−1 with an on/off ratio of 11. The shorter CH2 linker in (2) results in poorer orbital overlap, likely due to significant longitudinal and latitudinal slippage between molecules in the crystal lattice. As a consequence, no field-effect response was observed for the device fabricated from (2).

 Please wait while we load your content...
Please wait while we load your content...