Intermolecular anagostic interactions in group 10 metal dithiocarbamates†
Abstract
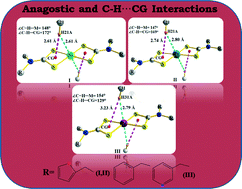
New functionalized homoleptic xanthate and dithiocarbamates of the form [M(L)2] (M = Ni(II), L = L1 (4-methoxyphenethylxanthate) 1, L2 (4-ethoxycarbonylpiperidine-1-dithiocarbamate) 2, L3 (N,N′-difurfuryldithiocarbamate) 3; M = Pt(II), L3, 4, M = Pd(II), L4 (N-benzyl-N′-3-methylpyridyldithiocarbamate) 5) have been synthesized and characterized using microanalyses, and their structures have been investigated by X-ray crystallography. All five complexes are centrosymmetric with the central metal in a distorted square planar structure; the distortion varies in the order Pt > Pd > Ni. In 3, 4 and 5 the ligand framework and crystal packing effects force the methylene proton on the substituents into close proximity with the metal centers, forming intermolecular C–H⋯M (M = Ni, Pt and Pd) anagostic interactions and generating a 1-D polymeric chain; 4 and 5 are the first examples containing Pt and Pd that exhibit such interactions. Similar anagostic interactions are also observed in 2 but are rather weaker. These interactions have been supported by theoretical calculations. The xanthate complex 1 displays unique S⋯S intermolecular interactions leading to a 1-D polymeric chain, while no significant intermolecular interactions involving the metal centres have been found. The supramolecular structures are sustained by weaker O⋯H, S⋯H, C–H⋯π and C–H⋯π (chelate, CS2M) interactions. 1 is weakly conducting (σrt = 1.39 × 10−7 S cm−1) but shows semiconductor behaviour in the 303–363 K range. The platinum complex 4 shows luminescent properties in solution.

 Please wait while we load your content...
Please wait while we load your content...