Z′ = 2 crystallization of the three isomeric piridinoylhydrazone derivatives of isosteviol†
Abstract
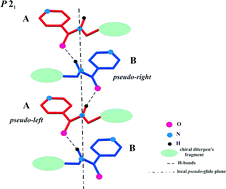
Crystallization of three diterpenoid isosteviol derivatives with a structural fragment of isoniazid (isonicotinic acid hydrazide) and its isomers – hydrazides of nicotinic and picolinic acids (structures 5, 6 and 7 respectively) was studied by single-crystal and powder XRD. These homochiral compounds crystallize in “Sohnke” space group P21 with two independent molecules. In crystals 5 and 6, an infinite H-bonded chain of alternating molecules A and B formed by N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interaction is observed, while only pairwise the same interactions between molecules A and B are realized in crystal 7. In the H-bonded associates of both types, the local (non-crystallographic) mirror-symmetry of nitrogen containing substituents in molecules A and B is observed. Such type of crystal packing – H-bonded chains or dimers formed by the N–H⋯O
C interaction is observed, while only pairwise the same interactions between molecules A and B are realized in crystal 7. In the H-bonded associates of both types, the local (non-crystallographic) mirror-symmetry of nitrogen containing substituents in molecules A and B is observed. Such type of crystal packing – H-bonded chains or dimers formed by the N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interaction through the glide plane – is the most typical packing in achiral compounds with a structural fragment of isoniazid and its isomers, as the CCDC analysis showed. Thus, structures 5–7 represent an interesting example of transferring a robust supramolecular mirror-symmetry motive from a racemic environment into a homochiral one, but inclusion of an “extra” molecule in a unit cell is the crystallographic “cost” of such transfer.
C interaction through the glide plane – is the most typical packing in achiral compounds with a structural fragment of isoniazid and its isomers, as the CCDC analysis showed. Thus, structures 5–7 represent an interesting example of transferring a robust supramolecular mirror-symmetry motive from a racemic environment into a homochiral one, but inclusion of an “extra” molecule in a unit cell is the crystallographic “cost” of such transfer.

 Please wait while we load your content...
Please wait while we load your content...