Modulating the solubility of sulfacetamide by means of cocrystals†
Abstract
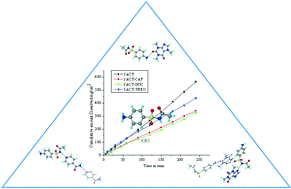
Sulfacetamide is a popular antibiotic prescribed for treating ocular infections. However, various physiological constraints are known to reduce its concentration at the site of action, thereby limiting its therapeutic efficacy. In this crystal engineering study, we report novel cocrystals of sulfacetamide with the objective to lower the solubility of the reference drug and improve its residence time at the site of action. Standard cocrystallization methods resulted in cocrystals with caffeine, isonicotinamide, theophylline, bipyridine and a salt with 4-aminopyridine. These crystalline forms were characterized by thermal, spectroscopic and diffraction techniques. In pH 7 phosphate buffer medium, sulfacetamide–caffeine cocrystal exhibited lower solubility (8.64 g L−1, 0.69 times) than the drug (12.5 g L−1). The dissolution of sulfacetamide–isonicotinamide and sulfacetamide–caffeine is 0.64 and 0.68 times lower, whereas sulfacetamide–theophylline is comparable to the reference drug. This study highlights a less explored application of pharmaceutical cocrystals to reduce the solubility and dissolution rate of the drug for improved therapeutic action.
- This article is part of the themed collection: Functional Co-crystals

 Please wait while we load your content...
Please wait while we load your content...