Ligand-controlled gold-catalyzed cycloisomerization of 1,n-enyne esters toward synthesis of dihydronaphthalene†
Abstract
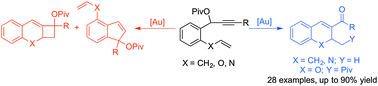
We describe herein a gold-catalyzed rearrangement of propargyl esters followed by allene–ene cyclization to afford substituted bicyclic [4.4.0] dihydronaphthalene compounds. This method is also applied to vinylethers and vinylamines of 1,7-enyne esters to form dihydroquinoline and dihydrobenzopyran structures. The basis of this transformation is the ligand-controlled preferential activation of the alkene over the allene, affording the desired aromatic bicyclic structures in moderate to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...