A catalytic asymmetric hetero-Diels–Alder reaction of olefinic azlactones and isatins: facile access to chiral spirooxindole dihydropyranones†
Abstract
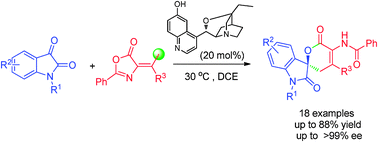
A catalytic asymmetric hetero-Diels–Alder (HDA) reaction has been achieved through hydrogen-bond directed γ-addition of olefinic azlactones to isatins. This methodology provides an efficient access to spirooxindole dihydropyranones in moderate to good yields and with excellent enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...