Effect of salt on the formation of salt-bridges in β-hairpin peptides†
Abstract
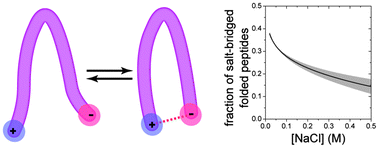
Salt-bridges ubiquitously form between oppositely charged moieties in proteins. Here we quantify changes in population of salt-bridged β-hairpin peptides due to added salt, and determine the thermodynamic driving forces and cooperativity of salt-bridge formation under these conditions. We find only a fraction of salt-bridged folded conformations at physiologically relevant salt concentrations.

 Please wait while we load your content...
Please wait while we load your content...