Bio-inspired synthesis of rare and unnatural carbohydrates and cyclitols through strain driven epimerization†‡
Abstract
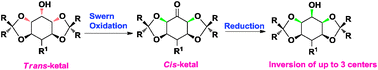
We report a bio-inspired, strain driven epimerization of trans-ketals to cis-ketals through an enolate intermediate. Swern oxidation of a hydroxyl group adjacent to a trans-ketal effects both oxidation and its epimerization to cis-ketal. This novel and general strategy allows inversion of up to three contiguous stereocenters and has been illustrated by the synthesis of several unnatural/rare isomers of carbohydrates/cyclitols from their naturally abundant isomers.

 Please wait while we load your content...
Please wait while we load your content...