Expanding the horizon of intermolecular trapping of in situ generated α-oxo gold carbenes: efficient oxidative union of allylic sulfides and terminal alkynes via C–C bond formation†
Abstract
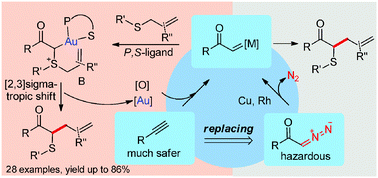
With a new P,S-bidentate phosphine as the ligand to gold(I), the α-oxo gold carbenes generated in situ via gold-catalyzed intermolecular oxidation of terminal alkynes were effectively trapped by various allylic sulfides, resulting in the formation of α-aryl(alkyl)thio-γ,δ-unsaturated ketones upon facile [2,3]sigmatropic rearrangements.

 Please wait while we load your content...
Please wait while we load your content...