Enantioresolution of dl-selenomethionine by thin silica gel plates impregnated with (−) quinine and reversed-phase TLC and HPLC separation of diastereomers prepared with difluorodinitrobenzene based reagents having l-amino acids as chiral auxiliaries
Abstract
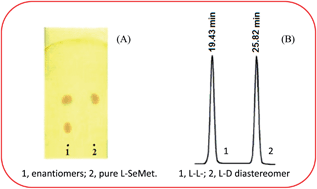
(−)-Quinine was used as a chiral selector by adopting three approaches in TLC for direct enantioresolution of DL-selenomethionine; these included impregnation of plate by chiral selector and its use as CMPA. Effect of pH, temperature and concentration of chiral selector on enantioresolution is studied. The spots were detected with ninhydrin and iodine vapors. Optically pure amino acids L-phenylalanine, S-benzyl-L-cysteine, and L-methionine were used to synthesize chiral derivatizing reagents (CDRs) based on fluorodinitrobenzene; these CDRs were used to synthesize diastereomers of DL-selenomethionine via nucleophilic substitution of the remaining fluorine atom in these reagents under microwave irradiation for 55 s at 75% (of 800 W) and also by stirring for 50 min at 45 °C. The diastereomers were separated by reversed-phase high-performance liquid chromatography on a C18 column with detection at 340 nm using aq. trifluoroacetic acid and acetonitrile as mobile phase under gradient elution while aq. triethylamine buffer and acetonitrile was successful as mobile phase for separation of diastereomers by reversed-phase thin layer chromatography. The conditions of derivatization and chromatographic separation were optimized. The method was validated for accuracy, precision, limit of detection and limit of quantification.

 Please wait while we load your content...
Please wait while we load your content...