Direct analysis of quaternary alkaloids by in situ reactive desorption corona beam ionization MS
Abstract
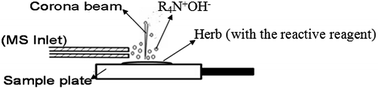
The direct detection of quaternary alkaloids by atmospheric pressure chemical ionization (APCI)-base ambient MS is difficult because of their poor volatility. In this study, a reactive protocol was developed for the in situ determination of quaternary alkaloids using desorption corona beam ionization (DCBI) mass spectrometry (MS). The model compounds of 8 quaternary alkaloids including sanguinarine, chelerythrine, cyclanoline, nitidine, coptisine, jatrorrhizine, berberine, palmatine and 2 tertiary alkaloids including protopine and allocryptopine were investigated in different states such as on a polytetrafluoroethylene (PTFE) plate, in raw herbal materials, and in silica gel. After various reactive reagents were studied, the mixture of saturated aqueous NaOH solution and CH3OH solvent (3 : 7, v/v) was selected as the optimized reactive reagent for the reactive DCBI-MS detection. All the target molecules can be detected with high sensitivity. On a PTFE plate the limits of detection were 0.0795, 0.1060, 0.4860, 0.9665, 0.8879, 0.3987, 0.5557, 0.4591, 0.0889, and 0.1929 mg L−1 for sanguinarine, chelerythrine, cyclanoline, nitidine, coptisine, jatrorrhizine, berberine, palmatine, protopine, and allocryptopine, respectively. The reactive protocol was also applied to the direct detection of raw herbal materials and thin layer chromatography successfully.

 Please wait while we load your content...
Please wait while we load your content...