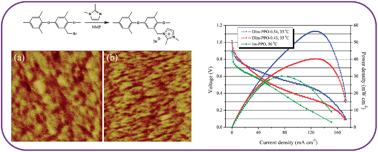
Novel anion exchange membranes (AEMs), based on poly(phenylene oxide) (PPO) chains linked to pendant 1,2-dimethylimidazolium (DIm) functional groups, have been prepared for evaluation in alkaline polymer electrolyte membrane fuel cells (APEFCs). Successful functionalisation of the PPO chains was confirmed using 1H-NMR and FT-IR spectroscopies. The ionic conductivities of the resulting DIm–PPO AEMs at 30 °C are in the ranges of 10–40 mS cm−1 and 18–75 mS cm−1 at 60 °C. The high ionic conductivities are attributed to the highly developed microstructures of the membranes, which feature well-defined and interconnected ionic channels (confirmed by atomic force microscopy, AFM, measurements). Promisingly, the ion-exchange capacities (IECs) of the DIm–PPO AEM are maintained after immersion in an aqueous KOH solution (2 mol dm−3) for 219 h at 25 °C; a previously developed monomethyl imidazolium PPO analogue AEM (Im–PPO) showed a significant decline in IEC on similar treatment. This reduction in undesirable attack by the OH− conducting anions is ascribed to an increase in steric interference and removal of the acidic C2 proton [in the monomethyl Im-groups] by the methyl group in the DIm cationic ring. Moreover, the maximum power densities produced in simple beginning-of-life single cell H2/O2 fuel cell tests increased from 30 mW cm−2 to 56 mW cm−2 when switching from the Im–PPO AEM (fuel cell temperature = 50 °C) to the DIm–PPO-0.54 AEM (fuel cell temperature = 35 °C) respectively (even with the use of lower temperatures).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...