Exploring suitable oligoamines for phantom ring-closing condensation polymerization with guanidine hydrochloride†
Abstract
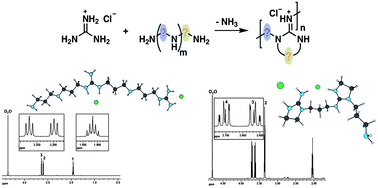
Different model compounds were applied in step-growth condensation reactions with guanidine hydrochloride in order to explore the scope of tri- and oligoamine applicability for phantom ring-closing condensation polymerization processes. The selective formation of five-membered rings was proven by APCI mass spectrometry, different one- and two-dimensional homo- and heteronuclear coupling NMR techniques and investigated via quantum chemical calculations. Furthermore the possibility of ring-closing reactions among guanidine hydrochloride and merely secondary amines was precluded. The obtained oligomers were exposed to antibacterial tests and exhibited a moderate activity towards Escherichia coli.

 Please wait while we load your content...
Please wait while we load your content...