Copper-mediated oxidative difluoromethylenation of aryl boronic acids with α-silyldifluoromethylphosphonates: a new method for aryldifluorophosphonates†
Abstract
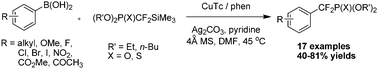
An unprecedented copper-mediated oxidative difluoromethylenation of aryl boronic acids with α-silyldifluoromethylphosphonates has been developed, allowing rapid access to a wide range of aryldifluorophosphonates containing various functional groups. This method provides a complementary and alternative method to Cu-mediated cross-couplings of aryl iodides with metalated difluoromethylphosphonates.
- This article is part of the themed collection: 崛起的中国—Hello from China!

 Please wait while we load your content...
Please wait while we load your content...