A series of six isostructural heterometallic trinuclear oxalate-bridged complexes of the formula (TDbenz)2(TsO)2[MII(H2O)2{(μ-ox)MIII(ox)2}2]·6H2O·2CH3OH (TDbenz = 1,3,5-tris[2-(1,3-diazolinium)]benzene; TsO = 4-methylbenzenesulfonate; ox = oxalate; MIII = Fe, MII = Mn (1), Fe (2), Co (3); MIII = Cr, MII = Mn (4), Fe (5), Co (6)) have been synthesized from (NH4)3[MIII(ox)3]·3H2O, the chloride salts of the divalent metal ions and the tosylate salt of 1,3,5-tris[2-(1,3-diazolinium)]benzene (trisamidinium). Whereas the crystal structures of compounds 2, 3, 4 and 5 have been investigated by single-crystal X-ray diffraction, the structures of 1 and 6 have been checked by X-ray powder diffraction. All six compounds are isostructural and crystallise in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The crystals are composed of discrete linear [MII(H2O)2{(μ-ox)MIII(ox)2}2]4− trinuclear bimetallic units, trisamidinium and tosylate ions and solvent molecules. The linear trinuclear unit is based on a central trans-diaquametal(II) entity connected to two [MIII(ox)3]3− (MIII = CrIII, FeIII) moieties through oxalate bridges. The divalent metal ions, surrounded by six oxygen atoms, adopt a distorted octahedral coordination geometry. The coordination sphere is composed of four oxygen atoms belonging to two oxalate ligands and two trans-coordinated water molecules. One of the oxalate ions is coordinated to the central metal centre whereas the other two oxalate ligands are non-bridging. In the crystal, intermolecular hydrogen bonds involving oxalate ligands, solvent molecules and the counter-ions form a complex 3D network. Variable-temperature magnetic susceptibility measurements indicate an antiferromagnetic interaction between the iron(III) and the metal(II) ions (J = −4.23, −6.73, −8.97 cm−1 for 1, 2 and 3 respectively) whereas this interaction is ferromagnetic when iron(III) is replaced by chromium(III) (J = +1.21, +2.20, +3.63 cm−1 for 4, 5 and 6 respectively). Moreover, the cobalt(II) derivatives exhibit high D values (D = 29.3 cm−1 for 3 and D = 27.4 cm−1 for 6).
space group. The crystals are composed of discrete linear [MII(H2O)2{(μ-ox)MIII(ox)2}2]4− trinuclear bimetallic units, trisamidinium and tosylate ions and solvent molecules. The linear trinuclear unit is based on a central trans-diaquametal(II) entity connected to two [MIII(ox)3]3− (MIII = CrIII, FeIII) moieties through oxalate bridges. The divalent metal ions, surrounded by six oxygen atoms, adopt a distorted octahedral coordination geometry. The coordination sphere is composed of four oxygen atoms belonging to two oxalate ligands and two trans-coordinated water molecules. One of the oxalate ions is coordinated to the central metal centre whereas the other two oxalate ligands are non-bridging. In the crystal, intermolecular hydrogen bonds involving oxalate ligands, solvent molecules and the counter-ions form a complex 3D network. Variable-temperature magnetic susceptibility measurements indicate an antiferromagnetic interaction between the iron(III) and the metal(II) ions (J = −4.23, −6.73, −8.97 cm−1 for 1, 2 and 3 respectively) whereas this interaction is ferromagnetic when iron(III) is replaced by chromium(III) (J = +1.21, +2.20, +3.63 cm−1 for 4, 5 and 6 respectively). Moreover, the cobalt(II) derivatives exhibit high D values (D = 29.3 cm−1 for 3 and D = 27.4 cm−1 for 6).
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The crystals are composed of discrete linear [MII(H2O)2{(μ-ox)MIII(ox)2}2]4− trinuclear bimetallic units, trisamidinium and tosylate ions and
space group. The crystals are composed of discrete linear [MII(H2O)2{(μ-ox)MIII(ox)2}2]4− trinuclear bimetallic units, trisamidinium and tosylate ions and 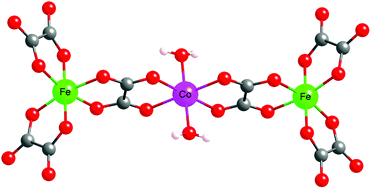

 Please wait while we load your content...
Please wait while we load your content...