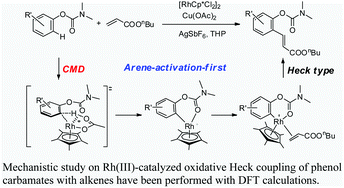
A systematic theoretical study on the Rh-catalyzed oxidative Heck-coupling of phenol carbamates with alkenes is carried out. Two possible mechanisms (i.e. arene activation-first and alkene activation-first mechanisms) are examined. As to the C–H activation step, four mechanisms including oxidative addition, electrophilic substitution, concerted metallation-deprotonation (CMD), and σ-bond metathesis are evaluated. The calculation results indicate that the arene activation-first mechanism is more favorable for the overall catalytic cycle. This mechanism involves three steps: arene C–H activation at the position ortho to the carbamate directing group affording a six-membered rhodiacycle intermediate, insertion of the alkene double bond into the Rh(III)–aryl bond, and a final β-H elimination step to release the product and re-generate the catalyst. The rate determining step of the overall catalytic cycle is the arene C–H activation step, which is found to proceed through the acetate-assisted CMD mechanism.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...