CO2/ethylene oxide copolymerization and ligand variation for a highly active salen–cobalt(iii) complex tethering 4 quaternary ammonium salts†
Abstract
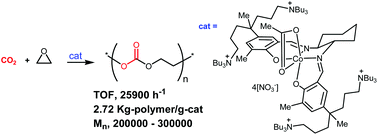
A cobalt(III) complex (1) of a salcy-type ligand tethering 4 quaternary ammonium salts, which is thought to act as a highly active catalyst for CO2/propylene oxide (PO) copolymerization, also shows high activity (TOF, 25 900 h−1; TON, 518 000; 2.72 kg polymer per g cat) and selectivity (>98%) for CO2/ethylene oxide (EO) copolymerization that results in high-molecular-weight polymers (Mn, 200 000–300 000) that have strictly alternating repeating units. The related cobalt(III) complexes 11–14 were prepared through variations of the ligand framework of 1 by replacing the trans-1,2-diaminocyclohexane unit with 2,2-dimethyl-1,3-propanediamine, trans-1,2-diaminocyclopentane, or 1,1′-binaphthyl-2,2′-diamine or by replacing the aldimine bond with ketimine. These ligand frameworks are thought to favour the formation of the cis-β configuration in complexation, and the formation of the cis-β configuration in 11–14 was confirmed through NMR studies or X-ray crystallographic studies of model complexes not bearing the quaternary ammonium salts. Complexes 11, 13, and 14, which adopt the cis-β configuration even in DMSO did not show any activity for CO2/PO copolymerization. Complex 12, which was constructed with trans-1,2-diaminocyclopentane and fluctuated in DMSO between the coordination and de-coordination of the acetate ligand as observed for 1, showed fairly high activity (TOF, 12 400 h−1). This fluctuating behaviour may play a role in polymerization. However, complex 12 did not compete with 1 in terms of activity, selectivity, and the catalyst cost.
- This article is part of the themed collection: Advances in metal-catalysed polymerisation and related transformations

 Please wait while we load your content...
Please wait while we load your content...