Evolution of biofunctional semiconductor nanocrystals: a calorimetric investigation†
Abstract
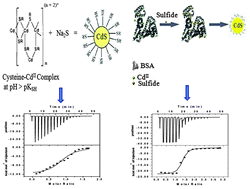
Semiconductor nanomaterials have found numerous applications in optoelectronic device fabrication and in platforms for drug delivery and hyperthermia cancer treatment, and in various other biomedical fields because of their high photochemical stability and size-tunable photoluminescence (PL). However, little attention has been paid to exploring the energetics of formation of these semiconductor nanoparticles. We demonstrate that formation of nanocrystals with biofunctionalization supported by widely used groups, BSA and cysteine, is an exothermic spontaneous process driven by enthalpy. The whole energetics of the reaction shows that formation of smaller particles is favored with lower synthesis temperature. Further, it is shown that the thermodynamics of nanoparticle formation is strongly influenced by the conformation of the protein matrix. We also demonstrate that protein supported formation of nanocrystals is thermodynamically more favorable compared to that involving smaller organic thiol groups. The favorable enthalpy of formation compensates unfavorable entropy, resulting in favorable Gibbs free energy. Thus, this study can open up new avenues for establishing a thermodynamic basis for the design of nanosystems with new and tunable properties.

 Please wait while we load your content...
Please wait while we load your content...