Raman enhancement by plasmonic excitation of structurally-characterized metal clusters: Au8, Ag8, and Cu8
Abstract
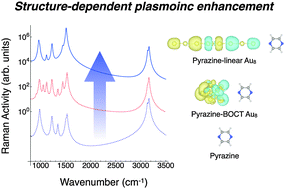
The optical responses of metal clusters, M8 (M = Au, Ag and Cu), are investigated by the linear response theory based on the density functional theory. Unlike sodium clusters [Yasuike et al., Phys. Rev. A, 2011, 83, 013201], the plasmonic excitations in the present metal clusters are strongly reduced by the background d-electron excitations, i.e., Landau damping. To avoid the reduction of plasmon intensity, the control of cluster structures is one of the promising strategies. We demonstrate that the plasmonic excitations of the linear clusters are partially decoupled with the background d-electron excitations and their intensities are much stronger than those of the three-dimensional bicapped octahedral isomers. The linear isomer gives a strong enhancement of the Raman vibrational spectrum of a
- This article is part of the themed collection: Plasmonics and spectroscopy

 Please wait while we load your content...
Please wait while we load your content...