Moisture-swing sorption for carbon dioxide capture from ambient air: a thermodynamic analysis
Abstract
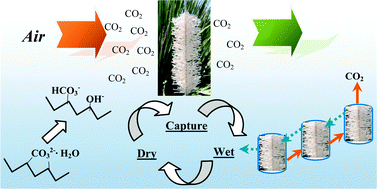
An ideal chemical sorbent for carbon dioxide capture from ambient air (air capture) must have a number of favourable properties, such as environmentally benign behaviour, a high affinity for CO2 at very low concentration (400 ppm), and a low energy cost for regeneration. The last two properties seem contradictory, especially for sorbents employing thermal swing adsorption. On the other hand, thermodynamic analysis shows that the energy cost of an air capture device need only be slightly larger than that of a flue gas scrubber. The moisture swing separation process studied in this paper provides a novel approach to low cost CO2 capture from air. The anionic exchange resin sorbent binds CO2 when dry and releases it when wet. A thermodynamic model with coupled phase and chemical equilibria is developed to study the complex H2O–CO2–resin system. The moisture swing behaviour is compatible with hydration energies changing with the activity of water on the resin surfaces. This activity is in turn set by the humidity. The rearrangement of hydration water on the resin upon the sorption of a CO2 molecule is predicted as a function of the humidity and temperature. Using water as fuel to drive the moisture swing enables an economical, large-scale implementation of air capture. By generating CO2 with low partial pressures, the present technology has implications for in situ CO2 utilizations which require low pressure CO2 gas rather than liquid CO2.

 Please wait while we load your content...
Please wait while we load your content...