Effect of A and B-site cations on surface exchange coefficient for ABO3 perovskite materials
Abstract
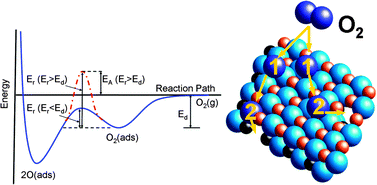
A novel approach, called isothermal isotope exchange (IIE), was applied to varying A- and B-site lanthanum manganites, ferrites, and cobaltites in the perovskite crystal structure in order to extract accurate surface exchange coefficients (k*). Pure electronic conductors revealed temperature dependent isotope exchange, while for mixed ionic and electronic conductors (MIEC) the extent of exchange was independent of temperature. MIEC materials have higher k* values than pure electronic conductors in the temperature range from 500–850 °C, demonstrating the importance of both electronic species and oxygen vacancies being present for surface exchange. Strontium doped perovskites exhibited opposite temperature dependencies to parent materials. Some perovskites exhibited an apparent negative

 Please wait while we load your content...
Please wait while we load your content...