Nitrogen dioxide at the air–water interface: trapping, absorption, and solvation in the bulk and at the surface
Abstract
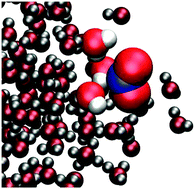
The interaction of NO2 with water surfaces in the troposphere is of major interest in atmospheric chemistry. We examined an initial step in this process, the uptake of NO2 by water through the use of molecular dynamics simulations. An NO2–H2O intermolecular potential was obtained by fitting to high-level ab initio calculations. We determined the binding of NO2–H2O to be about two times stronger than that previously calculated. From scattering simulations of an NO2 molecule interacting with a water slab we observed that the majority of the scattering events resulted in outcomes in which the NO2 molecule became trapped at the surface or in the interior of the water slab. Typical surface-trapped/adsorbed and bulk-solvated/absorbed trajectories were analyzed to obtain radial distribution functions and the orientational propensity of NO2 with respect to the water surface. We observed an affinity of the nitrogen atom for the oxygen in water, rather than hydrogen-bonding which was rare. The water solvation shell was less tight for the bulk-absorbed NO2 than for the surface-adsorbed NO2. Adsorbed NO2 demonstrated a marked orientational preference, with the oxygens pointing into the vacuum. Such behavior is expected for a mildly hydrophobic and surfactant molecule like NO2. Estimates based on our results suggest that at high NO2 concentrations encountered, for example, in some sampling systems, adsorption and reaction of NO2 at the surface may contribute to the formation of gas-phase HONO.

 Please wait while we load your content...
Please wait while we load your content...