Experimental and theoretical studies of the interaction of gas phase nitric acid and water with a self-assembled monolayer
Abstract
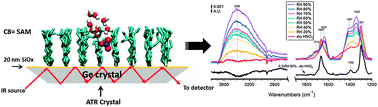
Nitric acid in air is formed by atmospheric reactions of oxides of nitrogen and is removed primarily through deposition to surfaces, either as the gas or after conversion to particulate nitrate. Many of the surfaces and particles have organic coatings, but relatively little is known about the interaction of nitric acid with organic films. We report here studies of the interaction of gaseous HNO3 with a self-assembled monolayer (SAM) formed by reacting 7-octenyltrichlorosilane [H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)6SiCl3] with the surface of a germanium infrared-transmitting attenuated total reflectance (ATR) crystal that was coated with a thin layer of silicon oxide (SiOx). The SAM was exposed at 298 ± 2 K to dry HNO3 in a flow of N2, followed by HNO3 in humid N2 at a controlled relative humidity (RH) between 20–90%. For comparison, similar studies were carried out using a similar crystal without the SAM coating. Changes in the surface were followed using Fourier transform infared spectroscopy (FTIR). In the case of the SAM-coated crystal, molecular HNO3 and smaller amounts of NO3− ions were observed on the surface upon exposure to dry HNO3. Addition of water vapor led to less molecular HNO3 and more H3O+ and NO3− complexed to water, but surprisingly, molecular HNO3 was still evident in the spectra up to 70% RH. This suggests that part of the HNO3 observed was initially trapped in pockets within the SAM and shielded from water vapor. After increasing the RH to 90% and then exposing the film to a flow of dry N2, molecular nitric acid was regenerated, as expected from recombination of protons and nitrate ions as water evaporated. The nitric acid ultimately evaporated from the film. On the other hand, exposure of the SAM to HNO3 and H2O simultaneously gave only hydronium and nitrate ions. Molecular dynamics simulations of defective SAMs in the presence of HNO3 and water predict that nitric acid intercalates in defects as a complex with a single water molecule that is protected by alkyl chains from interacting with additional water molecules. These studies are consistent with the recently proposed hydrophobic nature of HNO3. Under atmospheric conditions, if HNO3 is formed in organic layers on surfaces in the boundary layer, e.g. through NO3 or N2O5 reactions, it may exist to a significant extent in its molecular form rather than fully dissociated to nitrate ions.
CH(CH2)6SiCl3] with the surface of a germanium infrared-transmitting attenuated total reflectance (ATR) crystal that was coated with a thin layer of silicon oxide (SiOx). The SAM was exposed at 298 ± 2 K to dry HNO3 in a flow of N2, followed by HNO3 in humid N2 at a controlled relative humidity (RH) between 20–90%. For comparison, similar studies were carried out using a similar crystal without the SAM coating. Changes in the surface were followed using Fourier transform infared spectroscopy (FTIR). In the case of the SAM-coated crystal, molecular HNO3 and smaller amounts of NO3− ions were observed on the surface upon exposure to dry HNO3. Addition of water vapor led to less molecular HNO3 and more H3O+ and NO3− complexed to water, but surprisingly, molecular HNO3 was still evident in the spectra up to 70% RH. This suggests that part of the HNO3 observed was initially trapped in pockets within the SAM and shielded from water vapor. After increasing the RH to 90% and then exposing the film to a flow of dry N2, molecular nitric acid was regenerated, as expected from recombination of protons and nitrate ions as water evaporated. The nitric acid ultimately evaporated from the film. On the other hand, exposure of the SAM to HNO3 and H2O simultaneously gave only hydronium and nitrate ions. Molecular dynamics simulations of defective SAMs in the presence of HNO3 and water predict that nitric acid intercalates in defects as a complex with a single water molecule that is protected by alkyl chains from interacting with additional water molecules. These studies are consistent with the recently proposed hydrophobic nature of HNO3. Under atmospheric conditions, if HNO3 is formed in organic layers on surfaces in the boundary layer, e.g. through NO3 or N2O5 reactions, it may exist to a significant extent in its molecular form rather than fully dissociated to nitrate ions.

 Please wait while we load your content...
Please wait while we load your content...